Car owners know that small scratches may cause rusting, which will expand even under painted areas. Fighting rusting is a pretty hard task.
There are lots of methods that car keepers are using to prevent rusting. Many are using anti-rust coating, but it has shortcomings – success depends on how good it has been done. The rusted area needs to be cleaned with care and sometimes requires disassembling some pieces of a car. Such an operation requires a decent amount of time and constant control.
The car is always in a stressed environment: cold, salt, water, vibrations, stones, and other harsh conditions. There is still a chance of damaging surface coating. For constant prevention from further rusting, there is an electronic device used which requires only one installation.
Cathode rusting protection isn’t a new thing. It has been used for protecting various objects on top and underground. Ships are actively using cathode protection, which is aggressive in salty waters.
The idea is to use a special protector that melts in salty water instead of the protected metal. Water piping companies who use metal pipes first of all paints pipes with protection coating then wrap them in special tape and then at some distance places another metal electrode(Anode) protector and connect to it (+) and to the pipe(Cathode) (-). Because of the potential difference between electrodes, electrons flow to the cathode from the environment. On the anode side – electrons are freed, and so oxidation reaction starts while anode metal dissolves.
Cathode negative voltage has to be high enough to stop oxidation. For instance, to prevent iron from rusting or its alloys, only 0.1V to 0.2V needed. Higher voltages have no additional effect. The current density should be about 10 – 30mA/m2.
Because of oxygen buildup around the area of protection, an additional negative voltage accumulates. It is recommended periodically to disconnect the protection.
Cathode rusting protection device is very simple. You need two main blocks for the electronic part and protective electrodes. The electronics block may have an LED indicator, which indicates that the device is ON.
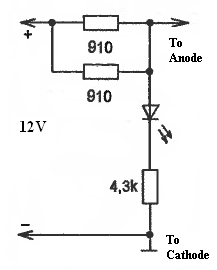
This device allows maintaining several decimal parts of volts on the car body enough to stop the rusting process and start destructing protective electrodes (Anodes). Anodes can be the same metals as car bodies (such as iron or aluminum) or other materials like magnetite and graphite. They have to be replaced every 4- 5 years. They usually are shaped as a square or circular plates with an area of 4 to 9cm2. It is essential to make sure that electrodes didn’t short-circuit. In the case of this, the LED indicator will stop indicating. LED light intensity may change because of changes in conductance variations because of environmental changes, e.g., a salted road in winter.






Very interesting, thank you very much!
Does this really work? seems very interesting indeed!
Good day! May I know what is mean ” 910 ” 2 units in your diagrams? is 910 ohm resitor?
ohm
i don’t think this can work for a car. a car is in air which will not conduct the ions at all. the examples of ship and pipes are connected with water and may therefore work.
This is defiantly not gonna work on car and don’t get your car screwed.